Quality is everyone’s responsibility. Historically, product quality is ensured by following the four eyes principle, or second person review, that requires two analysts to review, verify, and validate that the analytical instrument data created, analyzed, and reported was done in a compliant and scientifically correct workflow. However, these manual and tedious review workflows are quickly becoming the largest efficiency bottleneck for biopharma quality and manufacturing teams. Analysts and reviewers can easily spend half of their day fact-checking data against PDF golden batch readouts. Furthermore, these comparisons are subjective in nature as the analyst or reviewer is often visually comparing images from their chromatography data system (CDS) on one screen against a picture reference found in an “Integration Best Practices” PDF.
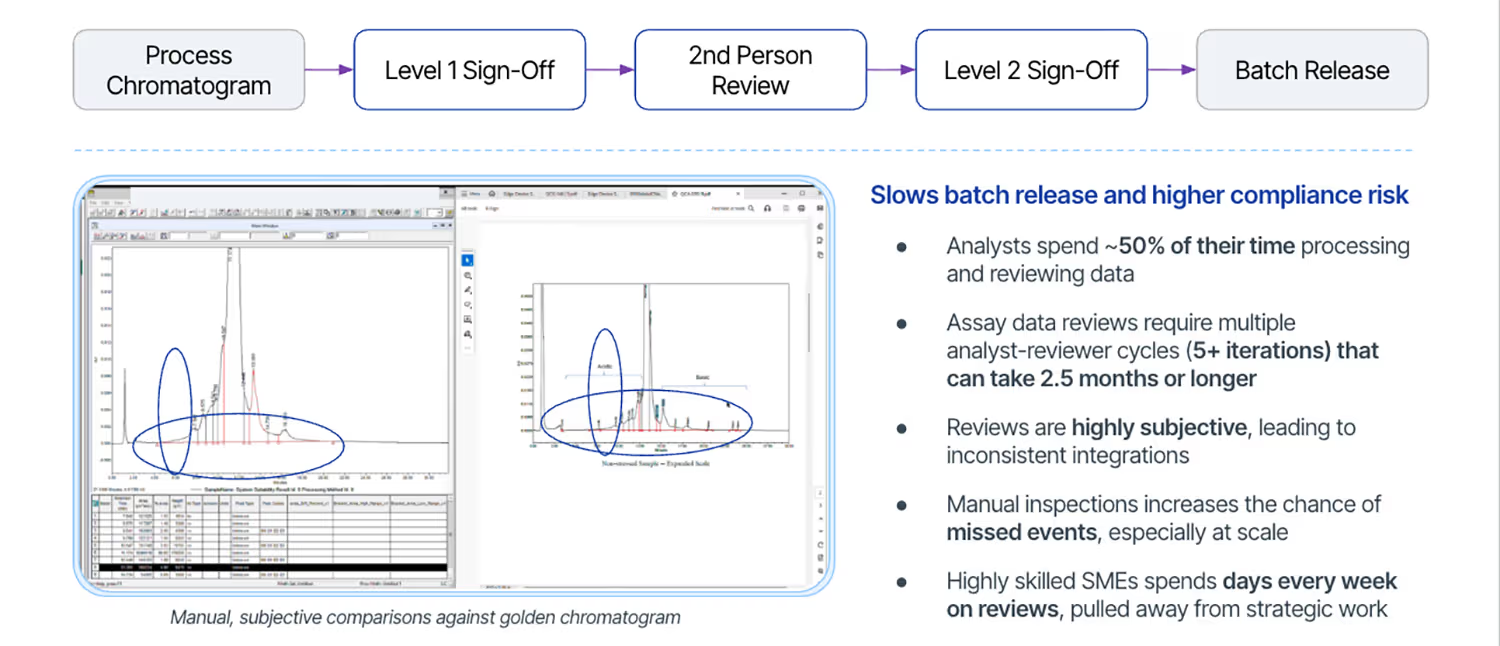
These subjective and manual data reviews invite scrutiny and compound compliance risk. A risk that can be detrimental to patients who are entitled to high-quality, safe, affordable and effective products. Failed batch releases not only impact patient safety, but they significantly increase the cost of manufacturing drug products and directly impact drug shortages. In 2020, the FDA Drug Shortages Task Force determined that 62% drug shortages from 2013 to 2017 were due to manufacturing and quality problems (FDA Drug Shortages Task Force, 2020, Shore, Brown, and Hopp. National Academies of Sciences, Engineering, and Medicine, Committee on Security of America’s Medical Product Supply Chain 2022). Single day shortages and batch recalls can quickly snowball in huge economic costs as biopharma daily operational costs can range from $600,000 to $8 million per day (Spott, 2024).
The manual, four-eyes principle review model cannot scale to rising quality and manufacturing demands, and does not align to strategic Lab of the Future roadmaps. Even regulatory guidelines like GMP Annex 11, ALCOA+, GLP, and ISO are written to support a digital, risk‑based review—both in what gets checked and how records are created and preserved at scale.
- Draft EU GMP Annex 11 (2025): Elevates risk‑based audit‑trail review and directs second‑person review to GMP‑relevant changes (e.g., manual integrations, parameter edits, sequence aborts), not routine logons or admin unlocks.
- ALCOA+, GLP, ISO: Meeting attributable, legible, contemporaneous, original, accurate at modern throughput requires validated electronic capture and exception‑centric verification; exhaustive manual checks inflate latency and error risk, so digital exception queues become the lowest‑risk path to compliance.
In short, regulators define the ‘what’ (risk‑bearing events) and the ‘how’ (validated, electronic review). But, what is the new digital, operational routine?
Review by Exception and the massive data challenge
Review by Exception is your essential strategy to navigate this transformation. It’s simple: use validated electronic systems to filter non-critical data, allowing your highly skilled experts to concentrate their efforts on scientific risk. The results are sharper compliance oversight, fewer missed deviations, and higher scientific vigilance.
Second‑person review will always be essential, but modern guidance permits the use of validated systems to flag anomalies and streamline review. Review by Exceptionaligns with ISPE, Annex 11, and 21 CFR Part 11 so that analysts and reviewers can focus on exceptions with confidence—that’s the game changer (McDowall LCGC 2020). Strategically this means that second-person review is prioritized for exceptions as opposed to fact checking every piece of quality data generated. Digital Review by Exception xecutes that routine comparison and review autonomously in the backend. Essentially, data review becomes an inherited byproduct from the Lab of the Future design.
Today’s QC environment can generate thousands of entries and touchpoints for a single batch. These touchpoints then multiply for stability studies that compare batches across lots, time points of weeks, months, and years, and numerous experimental stress conditions. Ultimately, the volume of data entries, manipulations, and decisions captured push manual review toward the impossible. As it stands today, manually reviewing complex lots can consume hundreds of hours and jam production. Analysts report spending half of their days performing single-person data review for complex biologic modalities that then require review and iteration with second-person data reviewers. Even where digital tools reduce some error modes by up to 60%, inefficiency persists because we still check everything instead of the risk.
Review by Exception is where AI earns its keep
RbE delivers tangible improvements by zeroing in on two critical areas:
- Audit Trail Review: Redirect second-person review from routine logins and administrative actions to GMP-critical events such as manual peak integrations, parameter changes, and method modifications—delivering the risk-based review Annex 11 expects.
- Chromatogram Profile Review: The system automatically flags tiny, high-risk deviations such as a subtle new degradation peak forming in a stability assay over 12 months, or an unexpected peak shift. This ensures your scientific integrity is guaranteed throughout the product lifecycle. Studies show this automation can cut review time on high-throughput plates from two hours to just 14 minutes.
The Vision: Review by Exception into real-time batch release
RbE is a prerequisite for achieving real-time batch release (RTBR)—the North Star for all quality leaders. Here’s how RbE transforms QC from a painful bottleneck into a value driver:
Building the Future: Your Digital Roadmap
Review by Exception requires a strategic and systematic shift from buying software to building a quality data foundry. The first step is globally harmonizing analytical instrument data and contextual experimental data to enforce structural consistency across sources and apply FAIR across platforms. Data harmonization is the foundation that empowers RbE capabilities. The combination of analytical quality data with digital SoPs, best integration guides, and audit checklists creates a digital fabric where systems can learn and infer common review patterns. These common review patterns and exception flagging provide the training material for building a robust, and scalable agentic framework for AI assistants to systematically identify and flag exceptions for analyst review.
Compliance is not a feel‑good initiative; it is an expected byproduct of a highly regulated industry that builds consumer trust and ensures patient safety. Review by Exception empowers teams to cut review effort by roughly half, and achieve batch release within hours to a few days of batch completion—compressing time‑to‑market while preserving control. Simultaneously , automated audit trail reviews ensure every flagged exception is reviewed and resolved with full traceability and transparency, establishing an always audit‑ready posture.
Review by Exception is the blueprint for the QC’s Lab of the Future. It ensures compliance, drives efficiency, and shifts experts from clerical checks to scientific leadership, paving the way to real-time batch releases.
The next post in this series will address the hidden costs and inherent risks of relying on traditional review workflows, demonstrating why RbE is the new benchmark for operational excellence.

